Nanoformulated Astaxanthin-polydopamine (ASX-PDA): Towards Disease-Modifying Therapeutics Development for Chronic Inflammatory Multiple Sclerosis Therapy (Acronym: nano-ASTADopa)
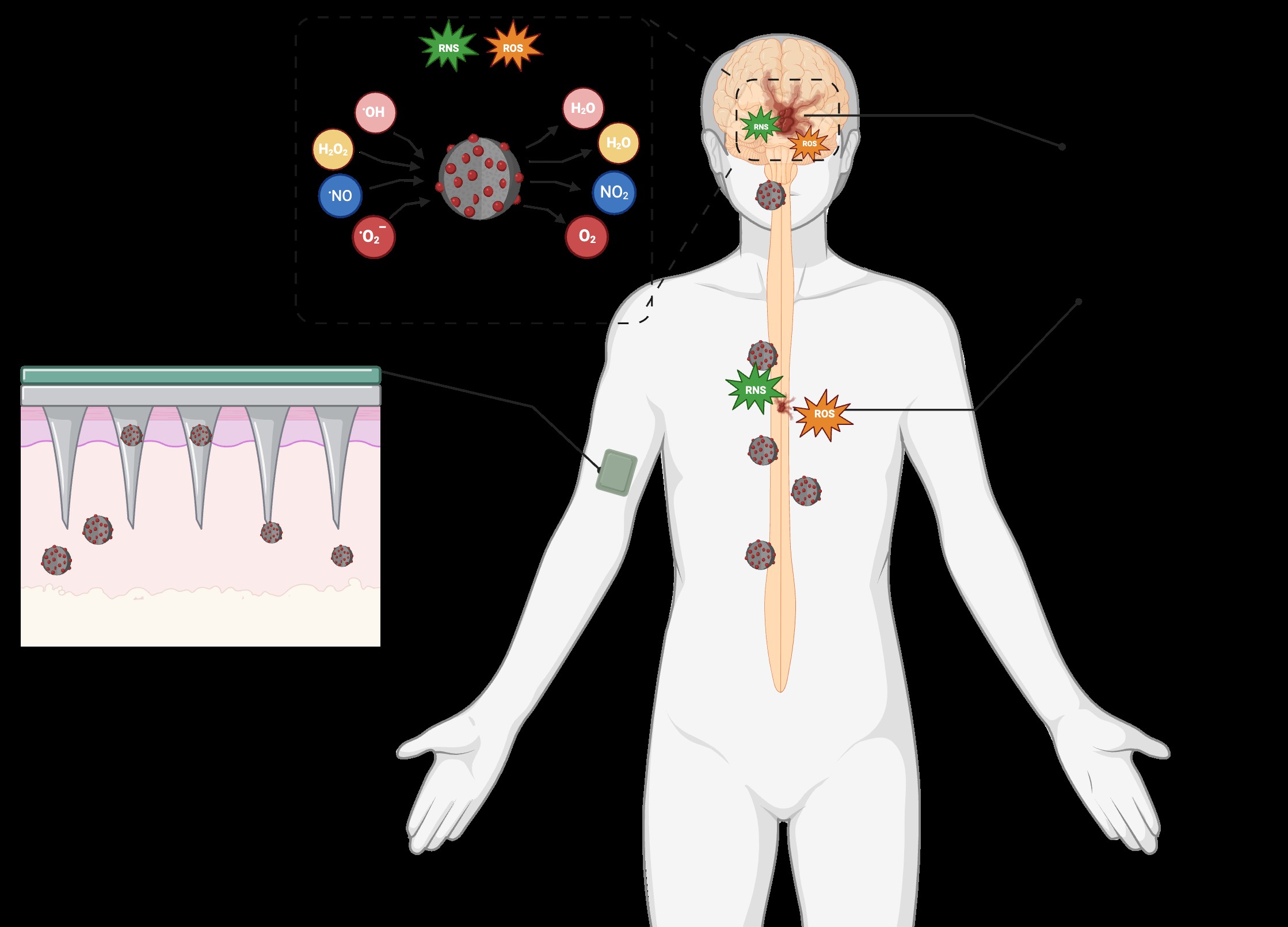
Astaxanthin (ASX) is an exceptionally potent antioxidant with clear mechanistic relevance for chronic inflammatory conditions such as multiple sclerosis (MS). Yet, its therapeutic potential remains practically underexplored due to its instability, poor aqueous solubility, and low bioavailability. Dr. Henke’s group succeeded in producing a natural, free-form, enantiomer-pure variant of ASX from Corynebacterium glutamicum, while Dr. Kabay’s team had been developing polydopamine (PDA)-based nanocarriers for controlled release and biological interfacing in parallel. A discussion between research groups uncovered a natural synergy between a bioactive molecule, ASX, available in high purity, and a nanoformulation engineering approach to stabilize and deliver it.
The resulting project, nano-ASTADopa, set out to engineer a nanoformulation in which natural ASX is loaded onto PDA nanoparticles. PDA offers aqueous dispersibility, biocompatibility, surface tunability, and radical-scavenging properties that complement ASX’s mode of action. The main objective was to demonstrate that PDA nanoparticles improve ASX stability and solubility and enable controlled release under fluctuating neuroinflammatory conditions characteristic of the progression of relapsing-remitting multiple sclerosis (RRMS). These are three essential prerequisites for turning the bioactive compound into an effective disease-modifying therapeutic candidate for RRMS.
Over the funding period, the team successfully extracted high-quality free-form ASX from the microbial process, synthesized size-controlled PDA nanoparticles (<200 nm, with a narrow distribution), and chemically modified the nanoformulation’s surface properties to enhance its colloidal stability. ASX was efficiently loaded onto the PDA matrix, achieving high encapsulation efficiency and maintaining ASX’s molecular integrity. The resulting ASX-PDA formulation showed significantly improved stability compared to free ASX. It demonstrated controlled release behavior in artificial interstitial fluid, indicating its suitability for intradermal or localized therapeutic delivery.
The project produced a proof-of-concept nanoformulation that overcomes the core pharmacological limitations of ASX in its extracted form, through bridging bioprocess engineering and materials science. These findings now serve as the scientific basis for a larger national or EU-funded research proposal, with the long-term goal of advancing the ASX-PDA nanoformulation toward preclinical testing as a disease-modifying therapy for RRMS.