Small: Nanosensors for Multiplex Detection of Contaminants in Food
Assuring the safety and quality of processed and primary food ingredients is an ongoing global challenge. Nanoparticle-based sensors or nanosensors provide a rapid and cost-effective solution for detecting food contaminants such as toxins and pathogens. In their review for the nanoscience & nanotechnology journal Small, Pierre Picchetti and colleagues show the potential of nanosensors to detect multiple targets in food samples with the required selectivity, sensitivity and speed for high throughput analysis. Moreover, this technology is robust, easy to use for non-specialized personnel, and can identify chemical substances in all states of aggregation, such as liquid or solid – with ultra-low detection limits.
With his KIT junior research group at the Institute of Nanotechnology, Pierre Picchetti focuses on developing synthetic nanomaterials that mimic enzymatic functions as well as nanocarriers that can release their cargo on demand in response to external stimuli.
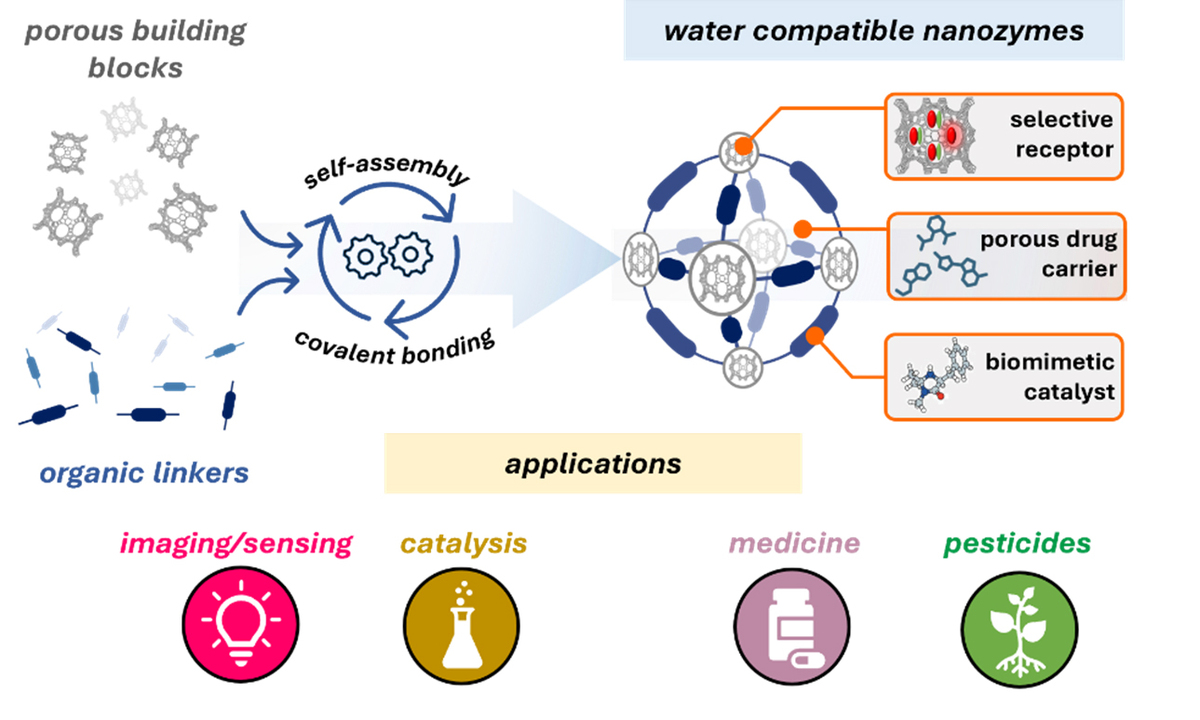
Nanozymes
“Our goal for the nano-enzymes – in short nanozymes – is to replicate key features of natural enzymes for the application in biosensing, catalysis, or biomedicine,” says Picchetti. “My broader vision is to establish these synthetic systems as functional analogues, capable of modulating cellular metabolism with high precision.” This approach could open the door to new in situ therapeutics synthesized directly within the cells, offering more targeted and effective treatments, including for cancer. To this end, the scientists use bio-compatible and water-soluble silica as basic material to which they add precisely assembled molecular building blocks. These building blocks are organic groups that serve a special purpose like keys to different locks. The combination of supramolecular and covalent chemistry, moreover, enables the fast, cost-effective and scalable synthesis of stable nanozymes, which exhibit reduced immunogenicity due to their non-protein nature. “A particularly promising avenue involves engineering nanozymes as intracellular catalysts that generate therapeutic agents in response to external stimuli,” Picchetti continues. While this approach already works in principle, challenges include precise targeting and the responsiveness to non-invasive stimuli such as ultrasound or infrared light.
Nanocarriers
Targeted and timely delivery of bioactive compounds in biological systems, such as animals or plants, remains a key challenge for the success of safer therapies and pesticide alternatives. One example was the Covid-19-vaccine where a messenger ribonucleic acid (mRNA) was embedded within a lipid-based carrier system as not to be biodegraded immediately upon local injection into the muscle. “We design inorganic nanocarriers that are both more cost-efficient and chemically robust,” explains Pierre Picchetti. “Drugs such as nucleic acids are much more effective if administered to the blood stream, thus, reaching most parts of an organism.” His group uses nucleic acids as a programmable structural component to create hybrid organic-inorganic materials. Thereby, they enhance the stability of the nucleic acids while simultaneously expanding the functionality of the inorganic components. The scientists currently explore these new materials for applications ranging from stimuli-responsive delivery systems for mRNA therapies to nucleic acid-based crop protection strategies. In the Chemical Society Reviews, Pierre Picchetti and colleagues discuss the application of supramolecular chemistry to plant science and agriculture, including in vivo in sensing and monitoring of plant processes and the preparation of safer and more effective supramolecular pesticides.
Representative Research Articles:
- P. Picchetti*, M. V. Balli, S. Baker, N. Manoj Kumar, P. Gruhs, L. Prodi, F. Biedermann*, Anal. Sens. 2024, 4, e202400025. DOI: 10.1002/anse.202400025
- P. Picchetti, S. Volpi, M. Sancho-Albero, M. Rossetti, M. D. Dore, T. Trinh, F. Biedermann, M. Neri, A. Bertucci, A. Porchetta*, R. Corradini*, H. Sleiman*, L. De Cola*, J. Am. Chem. Soc. 2023, 145, 22903-22912. DOI: 10.1021/jacs.3c04345
- N. Manoj Kumar, P. Gruhs, A. Casini, F. Biedermann, G. Moreno-Alcántar, P. Picchetti*, ACS Sens. 2023, 8, 2525-2532. DOI: 10.1021/acssensors.3c00008
- N. Manoj Kumar, P. Picchetti*, C. Hu, L. M. Grimm, F. Biedermann*, ACS Sens. 2022, 7, 2312-2319. DOI:10.1021/acssensors.2c00934
- P. Picchetti, G. Moreno-Alcántar*, L. Talamini, A. Mourgout, A. Aliprandi, L. De Cola*, J. Am. Chem. Soc. 2021, 143, 7681-7687. DOI: 10.1021/jacs.1c00444